Anode and Cathode in Electrolysis
The so-called anode effects and polarization and to avoid the difficulties related to the carbon anode 3-5. The cathode is negatively charged to remember how the cathode is negatively charged consider caThode T as a negative so it is easy to remember carhode as a negatively.

Electrolysis Of Copper Sulfate Solution With Impure Copper Anode And Pure Copper Cathode Copper Purification Technology Educat Pure Products Copper Solutions
Anode and Cathode.

. To make efficient use of electrical energy in the whole electrocatalysis conversion process the integrating of anode and cathode reactions plays a vital role. The table summarises the product formed at the anode during the electrolysis of different electrolytes. During electrolysis the anode loses mass as.
The cathode is the source of electrons or an electron donor. The electrolysis of copperII sulfate using copper electrodes is used to refine purify copper. Electrolysis of acidified water Water is a poor conductor of electricity but it does contain some hydrogen ions H and hydroxide ions OH -.
Bichlor Electrolysers Out-perform The Closest Alternative Technology. It is the electrodemetal plate that is connected to the positive terminal of the cell. Electrolysis converts electrical energy into chemical energy.
The cathode and anode the positive and negative. See reviews photos directions phone numbers and more for Albanese Electrolysis locations in Piscataway NJ. 485 44 votes.
Why is the cathode negative. The anode positive electrode is made from impure copper and the cathode negative electrode is made from pure copper. What are the products of electrolysis when CuSO4 is Electrolysed with Cu electrodes write anode and cathode reaction.
Electrolysis of Water. Twice as much as the anode reaction. One or Two 30 or 60-Minute Electrolysis Hair-Removal Sessions.
These ions are formed when a. Negatively charged ions move towards the anode. Reagents Dilute sulfuric acid.
Ad World-class Electrolysers And Electrode Coatings That Produce Chlor-alkali Products. The electrode at which oxidation occurs is called an anode and the one at which reduction occurs is called the cathode whether it is an electrolytic cell or a galvanic cell. Equipment Hoffman apparatus platinum electrodes AC-DC rectifier 100 DC house current.
Negative ion Element given off at. For example when a very dilute aqueous solution of NaCl is subjected to. We Are The Partner You Need.
Ad Offering Premium Products Exceptional Service For Over A Century. However limited attention has been paid to the study of cathode. The positively charged electrode in electrolysis is called the anode.
Offering Over 3000 Different Metal Compositions Shapes. The electrolysis of an aqueous solution of. Anode is electron deficient and hence the negative ions are.
Answer 1 of 3. Up to 10 cash back Discover Electrolysis Hair Removal Deals In and Near West Orange NJ and Save Up to 70 Off. The battery pumps electrons away from the anode making it.
Answer 1 of 5. During electrolysis the anode loses. The anode positive electrode is made from impure copper and the cathode negative electrode is made from pure copper.
An anode is an electrode where the electricity moves into. They are essentially opposite to the reactions occurring in galvanic cells and would not naturally occur without the application of. Whether hydrogen or.
A cathode is an electrode from where the electricity flows out or is given out. The electrolysis of acidulated water is considered to be an example of catalysis. Regardless of the nature of the ions only water molecules undergo electrolysis when dilute solutions are used.
A cathode is a negative-sided electrode. The impure Cu is the anodeA piece of pure Cu is used at the cathodeImpurities on the. B During the electrolysis of copper II sulphate solution using platinum as cathode and.
CHLORINSITU IIa is a compact on-site electrolysis system for the production of a low-chlorate hypochlorite solution from sodium chloride and electrical energy Equation 8 The chlorine.

Electrolysis Chemical Changes Electrochemistry Energy

Electrolysis Process On Passing Electric Current The Cations Move Towards The Cathode And Get Deposited Piscinas De Agua Salada Escuela De Natacion Piscinas

Look4chemistry Electrolytic Cell Electrochemistry Cell Positive And Negative

Physical Chemistry Positive Or Negative Anode Cathode In Electrolytic Galvanic Cell Chemistry Stack Electrochemistry Chemistry Classroom Teaching Chemistry
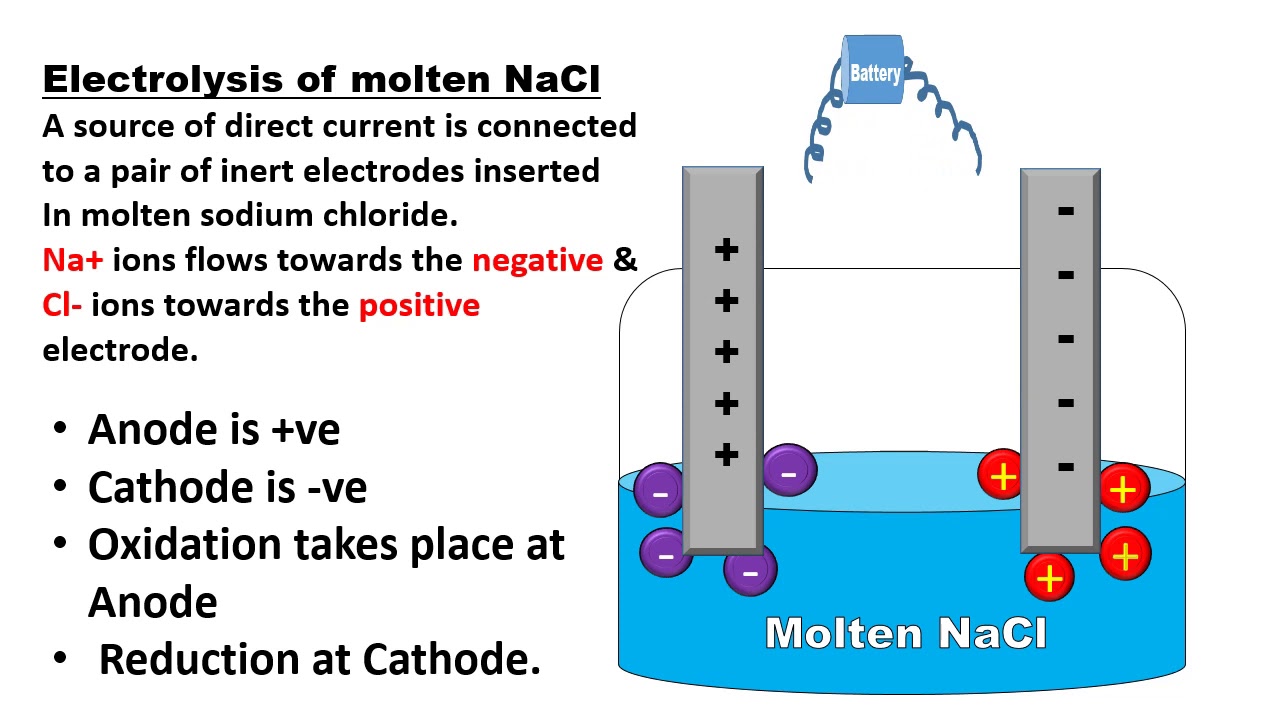
What Is Electrolytic Cell Electrochemistry Chemistry Basics Chemistry

Electrolytic Cell Electrolysis Of Nacl Electrochemistry Science Chemistry Electrochemical Cell
0 Response to "Anode and Cathode in Electrolysis"
Post a Comment